With kids heading back to school, the days growing shorter and the nights getting cooler, it can only mean one thing. We’re heading into cold and flu season. And with the COVID-19 pandemic still spreading throughout the world, it’s important to understand the difference between the various viruses and the vaccines available to prevent them. Here's what you need to know about protecting your members this cold and flu season:
- Influenza
- Pneumococcal
- Influenza and Pneumococcal Vaccine Pipeline Update
- COVID-19 and Other Noteworthy Vaccinations
Perspective on the Rx Pipeline: Vaccinations
Influenza
Influenza, commonly known as the flu, is a highly contagious respiratory virus (orthomyxoviridae family) often leading to symptoms such as fever, cough, sore throat, runny or stuffy nose, body aches, fatigue and headaches.
With social distancing, lessened travel and respiratory protection, the 2020/2021 flu season cases were unusually low; however, it is estimated that in a typical year in the United States, more than 200,000 patients are admitted to the hospital for influenza and there are approximately 36,000 flu-related deaths.[1, 2]
The Centers for Disease Control and Prevention (CDC) suggests everyone over six months of age get an influenza vaccine, particularly those at high risk of developing serious flu complications, such as adults over age 65, adults with chronic health conditions, children younger than two years of age, those with a body mass index (BMI) of 40 or higher, those with a weakened immune system, people who had a stroke, and pregnant women (for a full list, please see https://www.cdc.gov/flu/highrisk/index.htm). Influenza vaccinations are generally 40 to 60% effective in any given year.[3]
The CDC recommends getting the flu vaccine by the end of October, before community spread. It is important to note that it takes about two weeks after vaccination for the antibodies to develop. Getting the vaccine too early, such as July or August, may be associated with reduced protection later in the flu season.
There are three types of influenza virus, A, B and C; however, only A and B cause flu epidemics. For the 2021-2022 flu season, the World Health Organization (WHO) is recommending quadrivalent vaccines for the northern hemisphere, which includes the U.S.[4] Following are the recommended vaccine targets, updated annually, which may be available in different formulations, such as traditional injections, high-dose injections or intranasal spray (some vaccines may be trivalent):
Egg-Based Vaccines (currently used in the majority of vaccine products)
- A/Victoria/2570/2019 (H1N1) pdm09-like virus
- A/Cambodia/e0826360/2020 (H3N2)-like virus
- B/Washington/02/2019 (B/Victoria lineage)-like virus
- B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Cell or Recombinant-Based Vaccines
- A/Wisconsin/588/2019 (H1N1) pdm09-like virus
- A/Cambodia/e0826360/2020 (H3N2)-like virus
- B/Washington/02/2019 (B/Victoria lineage)-like virus
- B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
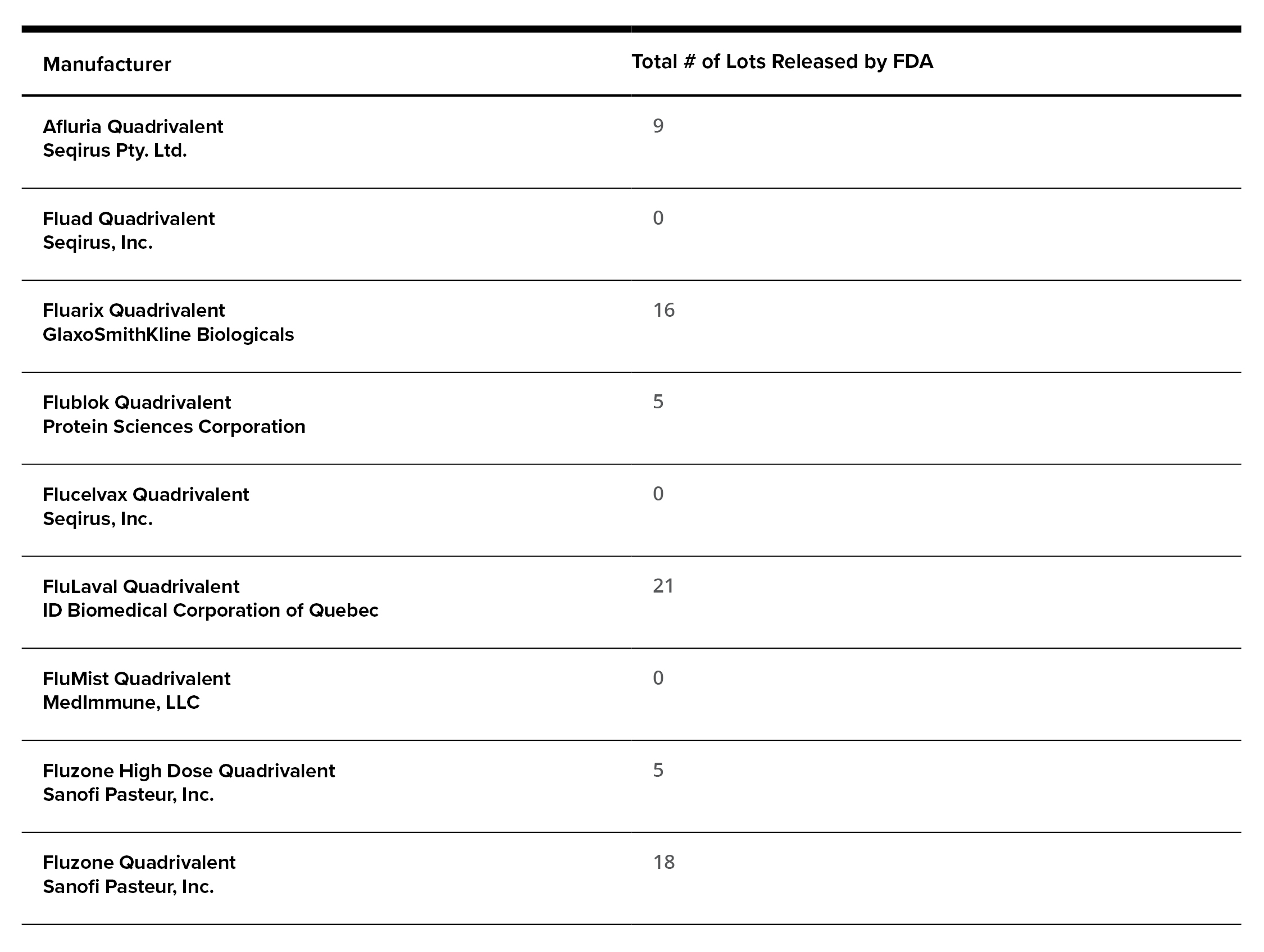
Flu Vaccination Availability in the U.S.: Various manufacturers have developed these vaccinations that have been approved by the FDA and are being released in the U.S. Following is a list of available flu vaccinations (updated 8/04/21):[5]
Pneumococcal
Pneumococcal disease is a very serious infection caused by streptococcus pneumoniae or pneumococcus bacteria. It can cause pneumonia (lung infection), meningitis (infection of the lining of the brain and spinal cord), bacteremia (blood infection), otitis media (middle ear infection) or sinusitis (sinus infection). Symptoms vary based on the type of infection, but many are similar to those of colds and flus. It is estimated to cause 150,000 hospitalizations each year in the United States and can lead to death.[6]
Pneumococcal vaccines are recommended by the CDC’s Advisory committee on Immunization Practices (ACIP) for both children and adults, particularly those at higher risk, such as children and adults with sickle cell disease, no spleen, HIV, cancer or other conditions that weaken the immune system (for a full list, please see https://www.cdc.gov/pneumococcal/about/risk-transmission.html).
Previously, the decision on what vaccine to administer was clear, as there were only two options, with consideration for patient’s age and comorbidities. In the last two months, two additional pneumococcal vaccines have come to market, Prevnar 20™ and Vaxneuvance™. Both vaccines are only indicated for use in adults; however, they cover additional pneumococcal serotypes than Prevnar 13® that are known to cause infection.[7]
Here is a review of the FDA-approved pneumococcal vaccines[8-11]:
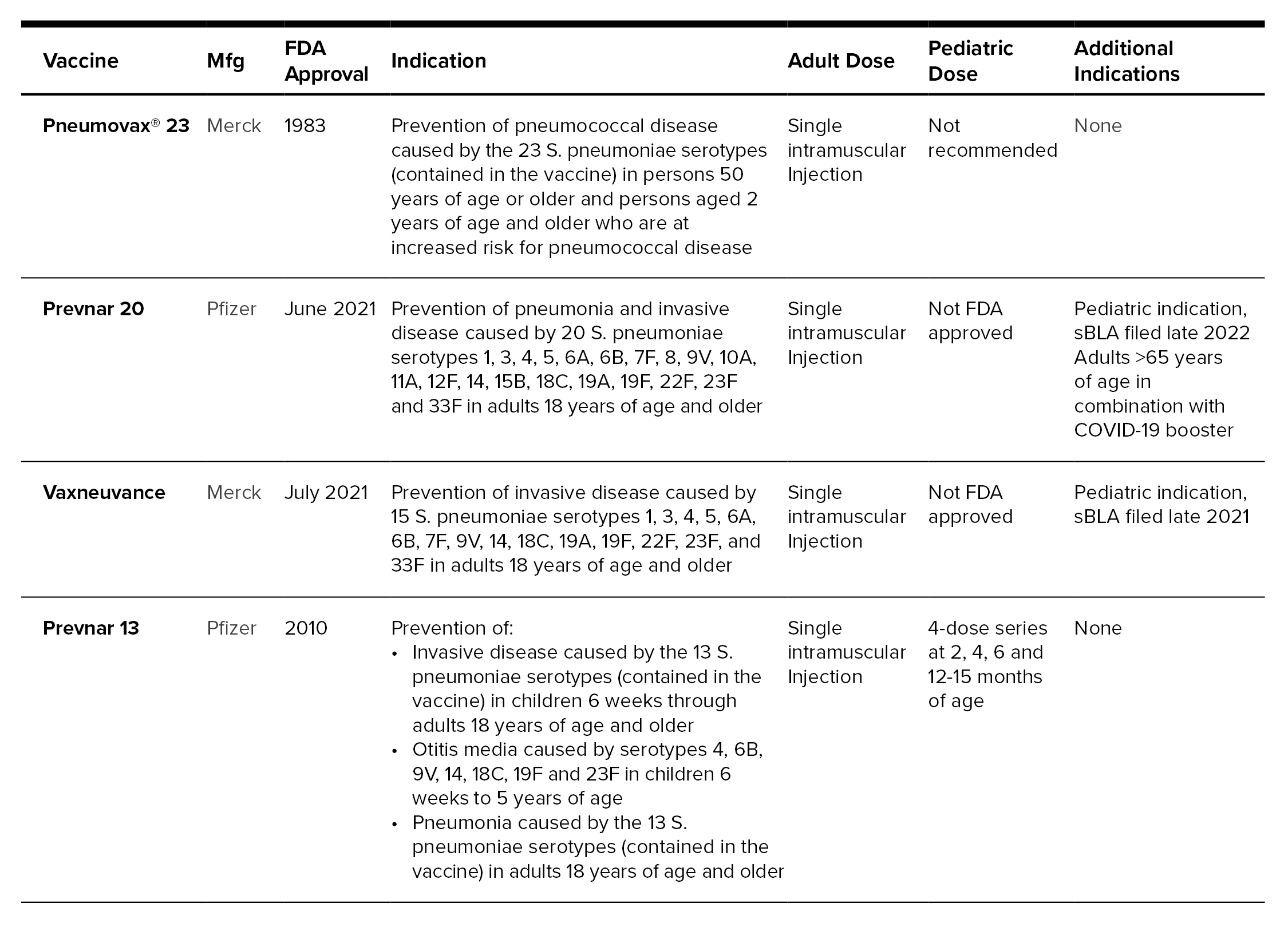
In 2019, ACIP updated recommendations for administration of Prevnar 13 and Pneumovax 23, removing routine use of Prevnar 13 in adults greater than 65 years of age. Adults 19-64 years of age who are immunocompromised, have cerebrospinal fluid leaks or cochlear implants require Pneumovax 23 and then at least one year later should receive Prevnar 13. For this population, an additional Pneumovax 23 vaccination is needed after 65 years of age, with the requirement that it has been eight weeks since receiving Prevnar 13, and it should have been at least five years from the previous Pneumovax 23 injection.[7]
Both Prevnar 20 and Vaxneuvance were non-inferior to Prevnar 13 in clinical trials and have additional serotypes for prevention. While additional FDA-approved vaccines are a positive update in the prevention of pneumococcal infections, the question of where Prevnar 20 and Vaxneuvance fit into the adult recommendations remains. ACIP will not meet until October 2021, to provide recommendations on these vaccines. The question is will these new vaccines replace Prevnar 13 in adults, target the high-risk population aged 19-64, or be part of a shared, individualistic decision with providers to determine if patients should have Prevnar 13, Prevnar 20 or Vaxneuvance. Additional ACIP recommendations will be needed if and when the FDA approves pediatric pipeline indications on these products as well.[7]

Influenza and Pneumococcal Vaccine Pipeline Update
There are a number of influenza and pneumococcal vaccines in the pipeline. Moderna is working on a seasonal flu vaccine that uses mRNA-based technology, which was introduced in the COVID-19 vaccines, and is planning to pursue human trials. Pfizer/BioNTech is also pursuing RNA technology for an influenza vaccination, claiming it could speed up the manufacturing process and remove the guess work of predicting the next season’s most common strains of influenza.[12] There are also additional vaccines in Phase II and III studies for pneumococcal that cover 23 and 24 serotypes. Following is a review of the influenza and pneumococcal vaccine pipeline:{12-15]
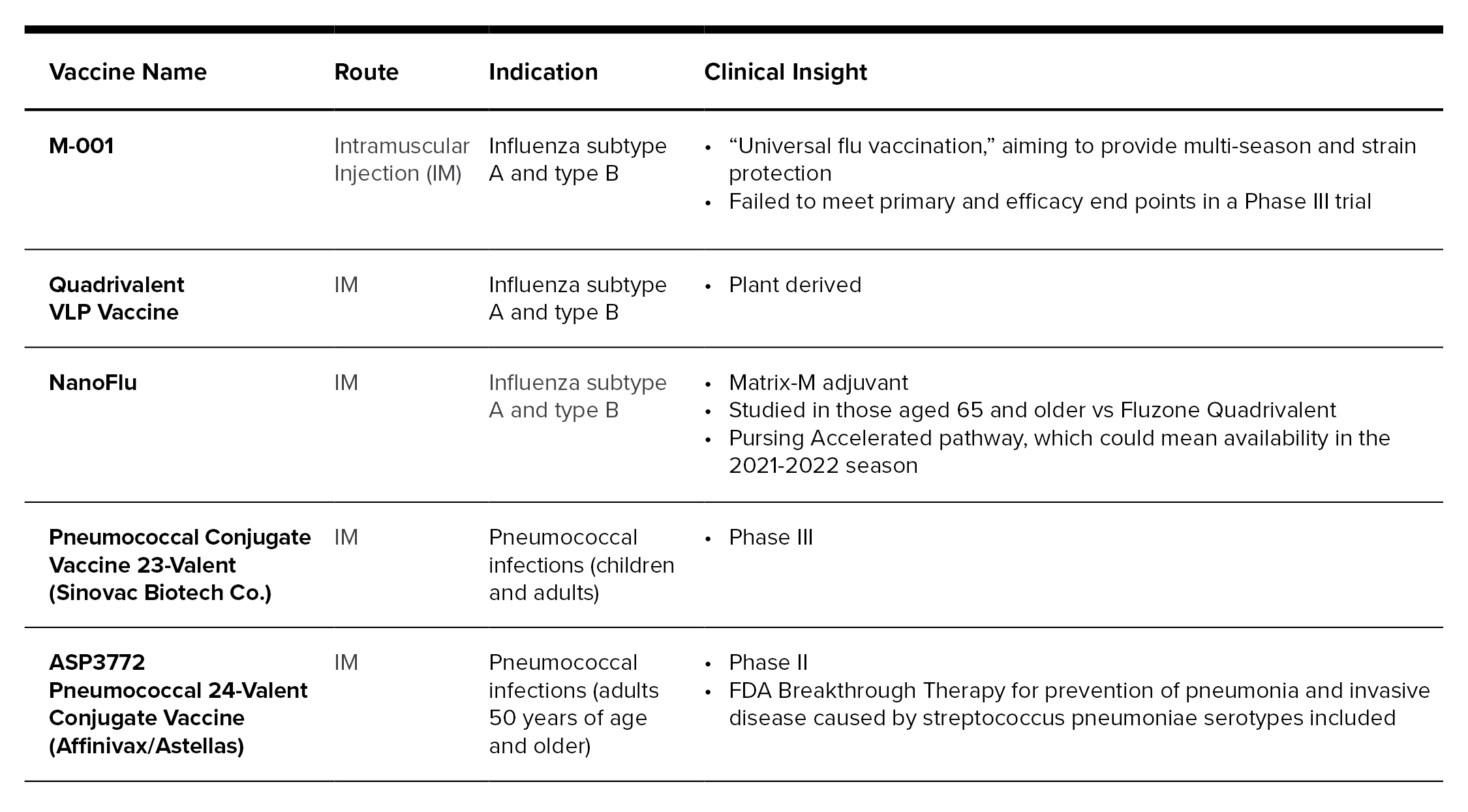
COVID-19 and Other Noteworthy Vaccinations
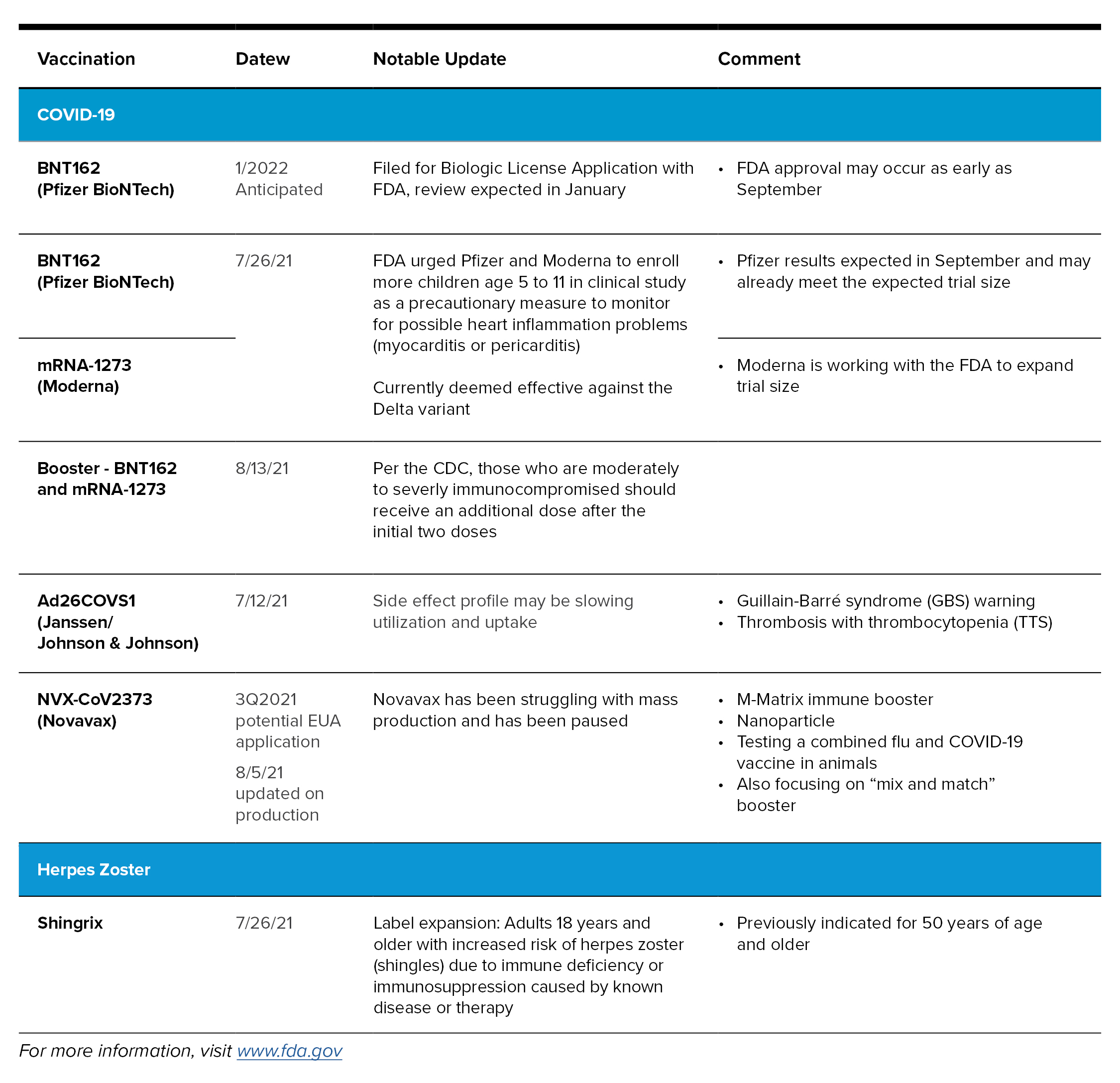
Adding to the mix is COVID-19, caused by a novel coronavirus that also has similar symptoms to colds and flus. There are three effective vaccines readily available in the United States that were given Emergency Use Authorization (EUA) from the FDA. Clinical trials are still being conducted for use of the vaccines in children and, without full FDA approval, there are still a number of people hesitant to get the vaccines. Variants of the virus continue to evolve and other countries throughout the world are still in need of vaccines. As of August 13, 2021, a COVID-19 vaccine booster is recommended only for immune compromised patients. A definition of what is considered moderately to severly immune compromised can be found at www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Following is a summary of COVID-19 and other miscellaneous vaccinations of note.
Impact to the Pharmacy Care Experience
Vaccinations are at the forefront of disease prevention efforts. Many vaccinations, especially those considered preventive, fall under the Affordable Care Act coverage requirement. Medicare coverage of vaccines is extensive and whether it is covered under Part D or Part B is dictated by the Centers for Medicare and Medicaid Services (CMS).
Pharmacy & Therapeutics Review and Formulary Strategies: Pharmacy benefit coverage strategies may be implemented when multiple products exist with matching clinical guidance and when regulations permit. Elixir’s Pharmacy and Therapeutics (P&T) committee will rigorously review each future FDA approval to assure clinically appropriate, safe and efficacious products are provided on our formulary.
Payer Action Plan
We believe encouraging members to adhere to and utilize pharmacy benefits when appropriate to receive vaccinations is an important clinical role pharmacy benefit managers and plan sponsors play. Elixir will continue to monitor the drug pipeline for new vaccinations and keep our clients apprised of updates. Our P&T committee will review any newly approved FDA products and update our clients when these products may be available for member utilization.
[1] Center for Disease Control. 2020-2021 (July 22,2021). Flu Season Summary. https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm Accessed August 6, 2021.
[2] National Center for Biotechnology Information (NCBI). Influenza Virus Biology and Epidemiology. https://www.ncbi.nlm.nih.gov/genome/viruses/variation/help/flu-help-center/influenza-virus-biology/ Accessed August 6, 2021.
[3] Center for Disease Control, National Center for Immunization and Respiratory Disease (NCIRD). (June 21, 2021). Who Needs a Flu Vaccine and When. https://www.cdc.gov/flu/prevent/vaccinations.htm#when Accessed August 6, 2021.
[4] World Health Organization (2021, February 26). Recommended composition of influenza virus vaccines for use in the 2021- 2022 northern hemisphere influenza season. https://cdn.who.int/media/docs/default-source/influenza/202102_recommendation.pdf?sfvrsn=8639f6be_3&download=true Accessed August 6, 2021.
[5] U.S. Food & Drug Administration (8/4/2021). Influenza Vaccine for the 2021-2022 Season. https://www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2021-2022-season Accessed August 6, 2021.
[6] Centers for Disease Control and Prevention. Fast Facts You Need to Know about Pneumococcal Disease. https://www.cdc.gov/pneumococcal/about/facts.html.
[7] Pneumococcal ACIP Vaccine Recommendations. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/pneumo.html Accessed July 30, 2021
[8] Pneumovax 23 (pneumococcal vaccine polyvalent) [prescribing information]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; December 2019.
[9] Prevnar 13 (pneumococcal conjugate vaccine [13-valent]) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; July 2019.
[10] Prevnar 20 (pneumococcal conjugate vaccine [20-valent]) [prescribing information]. Philadelphia, PA: Pfizer; June 2021.
[11] Vaxneuvance(pneumococcal conjugate vaccine [15-valent]) [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2021.
[12] Pfizer. New RNA technology Could Get the Flu Vaccine Right, Every Year. https://www.pfizer.com/news/featured_stories/featured_stories_detail/new_rna_technology_could_get_the_flu_vaccine_right_every_year Accessed August 6, 2021.
[13] Safety and Immunogenicity Study of 23-valent Pneumococcal Polysaccharide Vaccine in Healthy Children, Adults and Elderly. https://www.clinicaltrials.gov/ct2/show/NCT02451969?term=sinovac&cond=Pneumococcal+Infections&cntry=CN&draw=2&rank=1 Accessed July 30, 2021.
[14] Affinivax and Astellas Present Safety and Immunogenicity Data from Phase 2 Study of ASP3772, a Novel 24 valent MAPS™ Vaccine for Streptococcus pneumoniae. https://www.astellas.com/en/news/17066 Accessed July 30, 2021.
[15] Vaccines (2021). IPD analytics. https://www.ipdanalytics.com/. Accessed July 30, 2021.