Digital therapeutics (DTx) uses software programs to prevent, manage or treat diseases. They may be used with or without concurrent medications, with the goal of optimizing health results. Traditional digital wellness products, such as glucose monitors, track and record data.[1] DTx takes it a step further and helps manage a disease state. Some of the disease states DTx aims to treat or modify include substance use disorder, autism, major depressive disorder, insomnia and type II diabetes.[2] DTx may also provide behavioral or psychological services, such as cognitive behavioral therapy (CBT).
Perspective on the Rx Pipeline: Digital Therapeutics

Situation Summary
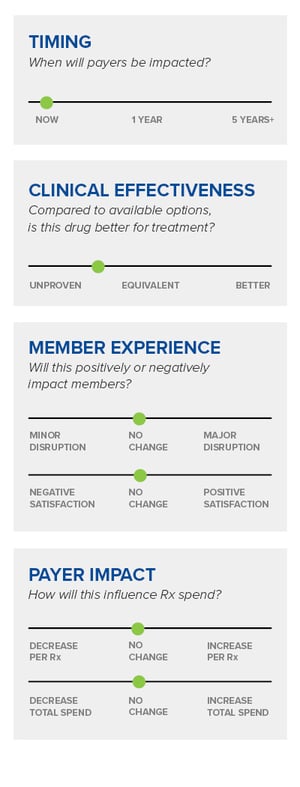
Digital therapeutics are often regulated by the FDA as medical devices, which differs from FDA-approved drugs and biologics. FDA approval of drugs generally includes the results of at least two clinical trials to ensure the findings, which are reviewed, along with the drug’s effects, by the Center for Drug Evaluation and Research (CDER).[3] The CDER then determines if the benefit of the drug outweighs the potential risk. Recent FDA-approved digital therapeutics approval pathways include:[4, 5, 6, 7]
- Pre-Market Notification 510(k) – This medical device approval process requires that the manufacturer demonstrate that the device is safe, effective and substantially equivalent to a legally marketed device by comparing it to another product.
- De Novo Classification – Created in 1997, this pathway is for low-to-moderate-risk novel or new devices where the manufacturer must provide reasonable assurance of safety and effectiveness for its intended use.
- Pre-Market Approval (PMA) – This is the most stringent regulatory approval pathway for Class III medical devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury. It is not often used for DTx at this time.
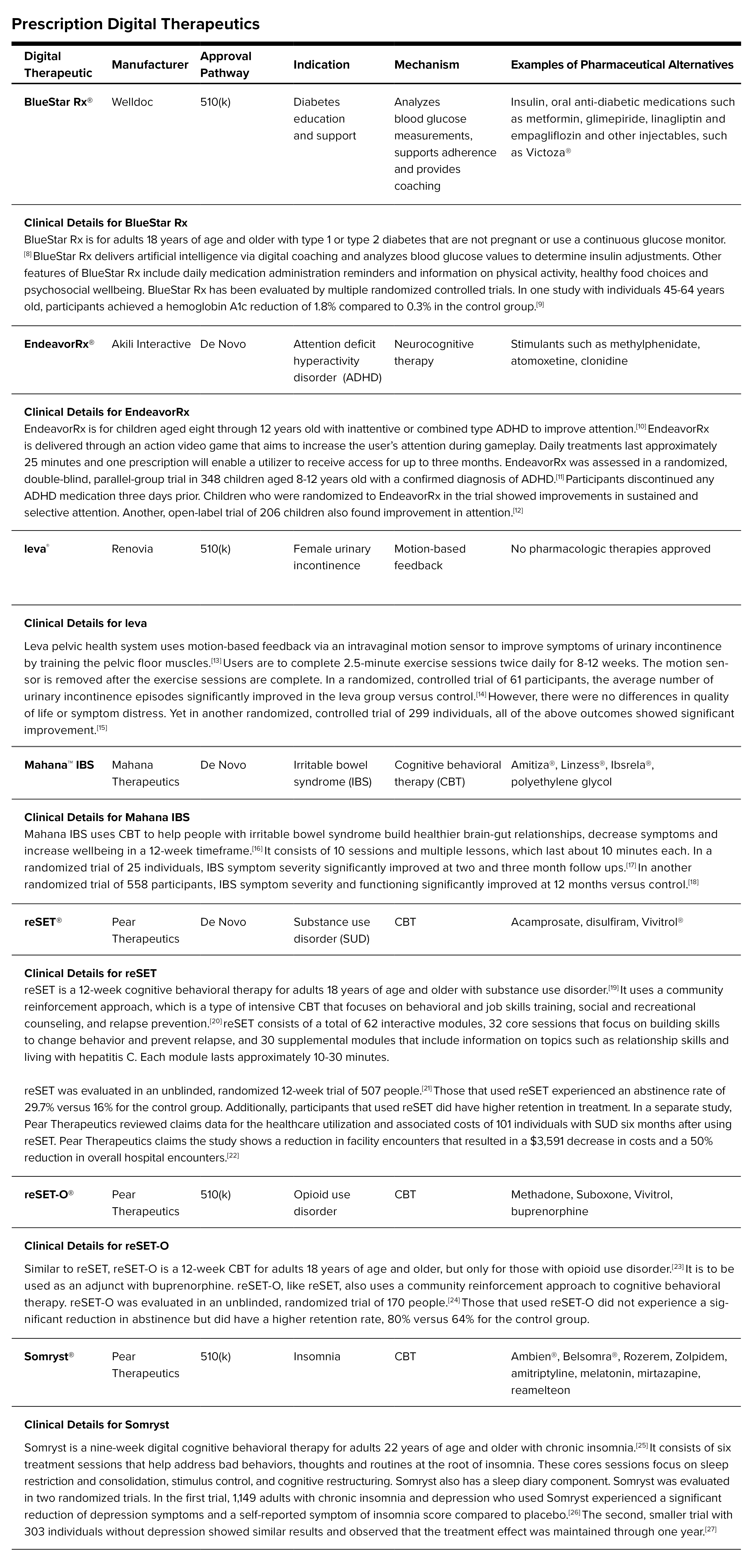
Approved Digital Therapeutics that Require a Prescription: The digital therapeutics market has exploded over the last few years and many are already in use at clinical practices and in homes. They can be sold over the counter or available by prescription. The robustness of clinical evidence to support digital therapeutics is overall lacking and inconsistent. Some products have shown positive outcomes with well-designed trials and results published in peer-reviewed journals, while others have not. In general, there is a lack of clinical guidance with these new technologies.
Following are several examples of FDA-approved digital therapeutics that require a prescription from a doctor:
Studies of Digital Therapeutics: There are many more applications (apps) and/or DTx available besides those listed above, and not all DTx are created equal. An article in Current Addiction Reports reviewed 59 opioid-related smartphone apps, studying their purpose, intended user, quality and popularity. The study found that 49% were related to treatment, 27% to prevention and 24% addressed opioid overdoses. Quality was rated using an eight-item screener from the American Psychiatric Association App Evaluation Model. Only one application met all eight evaluation criteria, the Pear’s ReSET-O (the only FDA-cleared app for treating opioid use disorder in the review). No other apps were associated with a clinical research study. Some apps did not have a transparent privacy policy and many had low adherence to clinical standards.[29,28]
The Institute for Clinical and Economic Review (ICER) released a report on digital therapeutics to treat opioid use disorder that stated that there were considerable limitations to provide an estimate of net health benefit for the digital therapeutics reSET-O, Connections and DynamiCare. The report noted that none of the DTx included were able to fully show how they could enhance abstinence or retention in treatment for those being treated with medication-assisted treatment (MAT). Although, the utilization of these digital therapeutics is unlikely to be harmful and might at least be comparable to MAT alone. While the clinical trials have limited supportive evidence, these digital apps may provide a small incremental benefit. ICER also did a cost-effectiveness analysis for reSET-O that showed its current price of $1,200 would meet cost-effectiveness. However, this was assuming that retention was held for five years. If this is not the case, then reSET-O’s price would need to be substantially lowered.[30]
These studies demonstrate that prescribers and payers should be aware of the range of effectiveness and utility of digital applications and DTx.
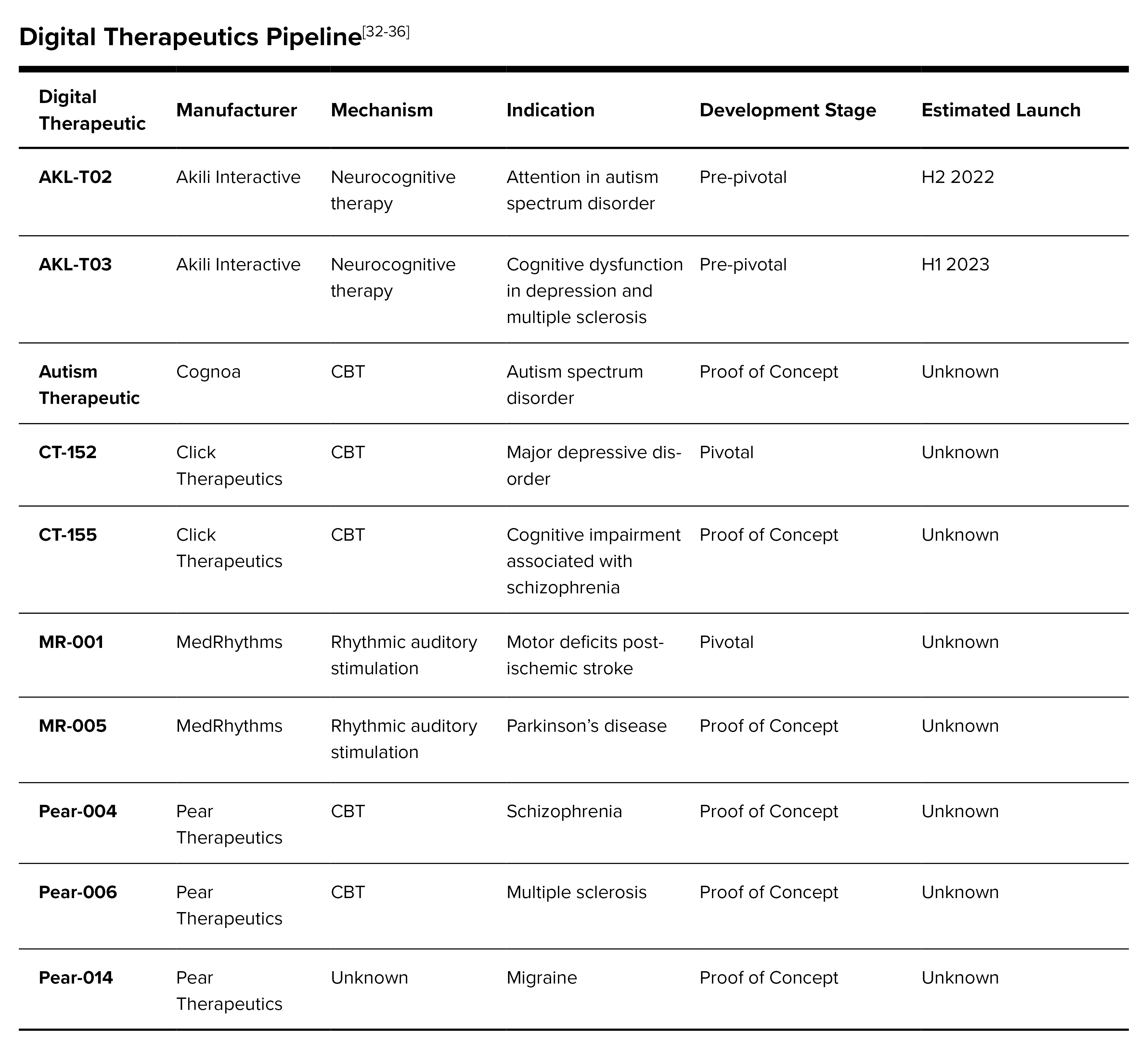
Digital Therapeutics in the Pipeline: There are multiple stages of development for DTx, but many pharmaceutical companies differ on nomenclature. Generally, these include discovery, proof of concept and pivotal.[31] The discovery stage is prior to clinical development where the mechanism of action and target population are defined and prototypes built. The proof-of-concept stage is early in the clinical development where the product is tested in human trials to prove that the concept is deserving of advancement to the next stage. And finally, the pivotal stage is where the product is tested in a trial designed to obtain market authorization from the FDA. There are a multitude of digital therapeutics in the pipeline. Following is a sampling of products in development from some of the more prominent digital therapeutics companies and indicated for various disease states.
DTx Guidance and Legislation: Digital therapeutics that require a prescription receive a unique device identifier, but these are not widely used under the pharmacy benefit today for billing or reimbursement. Some products may have been given a national drug code (NDC), but the FDA is looking to phase out NDC use. There is still much discussion about whether these products should live on the medical or prescription benefit for payers. For Medicare lines of business, the Centers for Medicare and Medicaid Services (CMS) recently created a new Healthcare Common Procedure Coding System (HCPCS) code for digital behavioral therapies. However, they have yet to give direction on how this code is to fit into the pharmacy or medical benefit. Also, there is proposed legislation known as the Access to Prescription Digital Therapeutics Act of 2022 intended to expand Medicare to cover prescription DTx.[37]
The National Council for Prescription Drug Programs (NCPDP) released the Background and Guidance for Using the NCPDP Standard for Digital Therapeutics version 1.1 in 2021, stating that in 2018 a DTx Task Group within the Maintenance and Control Work Group was created to attempt to determine if DTx products can be part of electronic data exchange between stakeholders on the prescription benefit.[38]
NCPDP’s proposed workflow consists of the following:
- Prescriber meets with patient and determines eligibility for DTx
- Prescription is sent to an intermediary to review prior authorization requirements and coverage
- Prescription is sent to the pharmacy (at the same time as #2)
- Once the prescription is sent to the pharmacy, it is filled and a claim is sent to the PBM using the NCPDP Telecommunication Standard Claim Billing Request
- The PBM would send the results back to the pharmacy with a notification of “paid” or “rejected”
- The pharmacy would collect appropriate copayment if the claim was paid by the PBM and the pharmacy is reimbursed for the claim by the payer
- Pharmacy dispenses to the patient, which may not be a physical product
An example of billing outside of this workflow is how Akili Interactive Labs, Inc. dispenses its EndeavorRx DTx for ADHD (noted above). Akili uses one pharmacy that sends a text message with a confirmation link once the prescription is completed and “filled.” Users then download the application on the App Store or Google Play and follow instructions. Refills may be requested.[39]
Many questions still exist with DTx as more information is gathered. DTx is software and may go through updates post-FDA clearance, how will this be regulated? Will the products be FSA/HSA eligible? What identifier will be used for payment and billing in the future? What type of security concerns exist over transferring information over the internet? Many require smartphones or tablets with certain operating platforms, for example, iOS 11 and Android 7 or higher; will this cause disparity in the non-technology savvy or those with less financial means?[2]
Impact to the Pharmacy Care Experience
Prescription Benefit Coverage: Currently, Elixir does not cover DTx under the prescription benefit due to many unknown questions about these medical devices. Validation of DTx clinical robustness may also be warranted. In general, not all medical devices may be covered under prescription benefit, as these are often approved under less stringent FDA pathways than FDA-approved drugs. Medical devices also may find better utility under a different healthcare benefit outside of prescription coverage. Safety concerns, updates and fluid payment models should be considered.
Pharmacy & Therapeutics Review and Formulary Strategies: Elixir will continue to monitor the DTx landscape. If pharmacy benefit coverage is deemed appropriate, Elixir’s Pharmacy & Therapeutics (P&T) committee will review the specific DTx product for formulary placement and evaluation of proper utilization management (ex., prior authorization and quantity limits) will be considered.
Payer Action Plan
Monitor the Drug Pipeline: Payers should stay up to date on recent and newly approved DTx. It’s important to remember not all digital products have clinical data or regulatory approval backing. And digital health products may encompass even more offerings with less regulation. Elixir will continue to monitor the pipeline of DTx that are FDA cleared with a prescription and keep our clients apprised of updates.
Commercial Coverage Considerations: Commercial payers should consider which benefit (medical or pharmacy) you want to cover these in the future, if coverage is desired. Elixir will continue to monitor the industry standard for coverage and evaluate if options are needed for coverage of DTx.
Monitor Medicare Mandates: Medicare payers should defer to CMS to determine what DTx should be covered. Elixir will watch for CMS guidance to see if and how digital therapeutics coverage is mandated.
[1] Lougheed T. (2019). How “digital therapeutics” differ from traditional health and wellness apps. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne, 191(43), E1200–E1201. https://doi.org/10.1503/cmaj.1095801
[2] Patel, N. A., & Butte, A. J. (2020). Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ digital medicine, 3(1), 159. https://doi.org/10.1038/s41746-020-00370-8
[3] U.S. Food & Drug Administration. (2022, August 8). Development & Approval Process| Drugs. https://www.fda.gov/drugs/development-approval-process-drugs
[4] U.S. Food & Drug Administration. (2020, March 13). Premarket Notification 510(k). https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k#:~:text=A%20510(k)%20is%20a,)(A)%20FD%26C%20Act).
[5] U.S. Food & Drug Administration. (2022, January 7). De Novo Classification Request. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/de-novo-classification-request
[6] U.S. Food & Drug Administration. (2019, May 16). Premarket Approval (PMA). www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma
[7] Johnston, J. L., Dhruva, S. S., Ross, J. S., & Rathi, V. K. (2020). Clinical Evidence Supporting US Food and Drug Administration Clearance of Novel Therapeutic Devices via the De Novo Pathway Between 2011 and 2019. JAMA internal medicine, 180(12), 1701–1703.
[8] BlueStar® and BlueStar Rx® - Digital Therapeutics Alliance. (2022, February 18). Digital Therapeutics Alliance; dtxalliance.org. https://dtxalliance.org/products/bluestar/
[9] Quinn, C. C., et al. (2016). Mobile Diabetes Intervention for Glycemic Control in 45- to 64-Year-Old Persons With Type 2 Diabetes. Journal of applied gerontology : the official journal of the Southern Gerontological Society, 35(2), 227–243. https://doi.org/10.1177/0733464814542611
[10] EndeavorRx®. Digital Therapeutics Alliance. (2022, February 18). https://dtxalliance.org/products/endeavor/
[11] Kollins, S. H., et al. (2020). A novel digital intervention for actively reducing severity of pediatric ADHD (STARS-ADHD): a randomised controlled trial. The Lancet. Digital health, 2(4), e168–e178. https://doi.org/10.1016/S2589-7500(20)30017-0
[12] Software Treatment for Actively Reducing Severity of ADHD as Adjunctive Treatment to Stimulant - Full Text View - ClinicalTrials.gov. (n.d.). Software Treatment for Actively Reducing Severity of ADHD as Adjunctive Treatment to Stimulant - Full Text View - ClinicalTrials.Gov; clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03649074
[13] leva® - Digital Therapeutics Alliance. (2022, March 21). Digital Therapeutics Alliance; dtxalliance.org. https://dtxalliance.org/products/leva/
[14] Weinstein, M. M., et al. (2022). Multicenter Randomized Controlled Trial of Pelvic Floor Muscle Training with a Motion-based Digital Therapeutic Device versus Pelvic Floor Muscle Training Alone for Treatment of Stress-predominant Urinary Incontinence. Female pelvic medicine & reconstructive surgery, 28(1), 1–6. https://doi.org/10.1097/SPV.0000000000001052
[15] Weinstein, M. M., Dunivan, G., Guaderrama, N. M., & Richter, H. E. (2022). Digital Therapeutic Device for Urinary Incontinence: A Randomized Controlled Trial. Obstetrics and gynecology, 139(4), 606–615. https://doi.org/10.1097/AOG.0000000000004725
[16] IBS Treatment | Mahana Therapeutics. (n.d.). IBS Treatment | Mahana Therapeutics; www.mahanatx.com. https://www.mahanatx.com/treatments/ibs
[17] Owusu, J. T., et al (2021). A pilot feasibility study of an unguided, internet-delivered cognitive behavioral therapy program for irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, 33(11), e14108. https://doi.org/10.1111/nmo.14108
[18] Everitt, H. A., et al (2019). Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut, 68(9), 1613–1623. https://doi.org/10.1136/gutjnl-2018-317805
[19] reSET® - Digital Therapeutics Alliance. (2022, February 16). Digital Therapeutics Alliance; dtxalliance.org. https://dtxalliance.org/products/reset/
[20] Meyers, R. J., Roozen, H. G., & Smith, J. E. (2011). The community reinforcement approach: an update of the evidence. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 33(4), 380–388.
[21] Campbell, A. N., et al (2014). Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. The American journal of psychiatry, 171(6), 683–690. https://doi.org/10.1176/appi.ajp.2014.13081055
[22] Shah, N., Velez, et al (2022). Real-World Reductions in Healthcare Resource Utilization over 6 Months in Patients with Substance Use Disorders Treated with a Prescription Digital Therapeutic. Advances in therapy, 1–11. Advance online publication. https://doi.org/10.1007/s12325-022-02215-0
[23] reSET-O® - Digital Therapeutics Alliance. (2022, February 16). Digital Therapeutics Alliance; dtxalliance.org. https://dtxalliance.org/products/reset-o/
[24] Christensen, D. R., et al (2014). Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. Journal of consulting and clinical psychology, 82(6), 964–972. https://doi.org/10.1037/a0037496
[25] Somryst® - Digital Therapeutics Alliance. (2022, February 16). https://dtxalliance.org/products/somryst/
[26] Christensen, H., et al (2016). Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. The lancet. Psychiatry, 3(4), 333–341. https://doi.org/10.1016/S2215-0366(15)00536-2
[27] Ritterband, L. M., et al (2017). Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA psychiatry, 74(1), 68–75. https://doi.org/10.1001/jamapsychiatry.2016.3249
[28] Wehrwein, P. (2021). Digital Therapetuics Shaping the Future of Care. MHE. 31(12). https://www.managedhealthcareexecutive.com/view/digital-therapeutics-shaping-the-future-of-care
[29] Vilardaga, R., et al (2020). Review of Popularity and Quality Standards of Opioid-Related Smartphone Apps. Current addiction reports, 7(4), 486–496. https://doi.org/10.1007/s40429-020-00344-6
[30] Institute for Clinical and Economic Review (ICER). Digital Health Technologies as an Adjunct to Medication Assisted Therapy for Opioid Use Disorder. November 6, 2020. https://icer.org/wp-content/uploads/2020/08/ICER_Digital_Therapeutics_for_OUD_Evidence_Report.pdf
[31] Product Pipeline - Pear Therapeutics (US). (n.d.). Pear Therapeutics (US); peartherapeutics.com. https://peartherapeutics.com/science/product-pipeline/
[32] Product Development — Akili Interactive. (n.d.). Akili Interactive; www.akiliinteractive.com. www.akiliinteractive.com/product-development
[33] Pipeline - Cognoa. (2022, June 9). Cognoa; cognoa.com. https://cognoa.com/our-science/pipeline/
[34] Developing DTx | Products & Pipeline. (n.d.). Click Therapeutics; www.clicktherapeutics.com. https://www.clicktherapeutics.com/products/
[35] Technology | MedRhythms. (2022, June 8). MedRhythms; medrhythms.com. https://medrhythms.com/technology/#pipeline
[36] Product Pipeline - Pear Therapeutics (US). (n.d.). Pear Therapeutics (US); peartherapeutics.com. https://peartherapeutics.com/science/product-pipeline/
[37] United States Congressman Mike Thompson. (2022, March 10). Thompson Introduces Bipartisan, Bicameral Bill to Ensure Access to Prescription Digital Therapeutics. https://mikethompson.house.gov/newsroom/press-releases/thompson-introduces-bipartisan-bicameral-bill-to-ensure-access-to
[38] National Council for Prescription Drug Programs (NCPDP) (2021, August). Background and Guidance for Using the NCPDP Standards for Digital Therapeutics v1.1
[39] EndeavorRx®. (2022) Want to get a Prescription? https://www.endeavorrx.com/getting-a-prescription/



