Atopic dermatitis (AD) is a skin condition that affects approximately 9.6 million children in the United States, with an estimated 16.5 million continuing to suffer from the condition as adults.[1] While it is more prevalent in children, adult onset of AD can also occur. AD may be viewed as just a rash, but it can have a profound effect on a person’s quality of life. In a recent study, 70.5% of participants with AD reported severe, unbearable itching that can impact sleep and other daily activities.[1] As the pipeline of prescription options to help treat this chronic condition continues to expand, it could also have an impact on healthcare costs.
Perspective on the Rx Pipeline: Atopic Dermatitis

Situation Summary
 AD is a chronic condition that can flare up with environmental factors, triggers or other comorbidities (e.g., eczema, asthma, food allergies or allergic rhinitis).[2] It can vary in severity and presentation, such as irritated skin patches that are swollen and itchy. Over time, there can be skin thickening or fissuring in areas of chronic scratching, and some patches can be at risk for viral or bacterial infections.[3] Diagnosis is based on history and skin lesion evaluation. Some data has shown that earlier age of onset could determine severity and longevity into adulthood.[4]
AD is a chronic condition that can flare up with environmental factors, triggers or other comorbidities (e.g., eczema, asthma, food allergies or allergic rhinitis).[2] It can vary in severity and presentation, such as irritated skin patches that are swollen and itchy. Over time, there can be skin thickening or fissuring in areas of chronic scratching, and some patches can be at risk for viral or bacterial infections.[3] Diagnosis is based on history and skin lesion evaluation. Some data has shown that earlier age of onset could determine severity and longevity into adulthood.[4]
Treatment Options: While there are a variety of both pharmacological and non-pharmacological treatment options for AD, moderate to severe forms of the condition are often resistant to therapy.
Perfumes, dyes and other ingredients in a daily skin care routine can trigger AD, so the most standard non-pharmacological approach of doctors is to teach good skin care habits (e.g., application of moisturizer, warm bath or a shower using non-soap cleanser) and trigger avoidance, including removal of scented detergents or soaps. Adding in antibiotics and even antiseptics may become necessary as the number of patches and symptoms increase or worsen.[2]
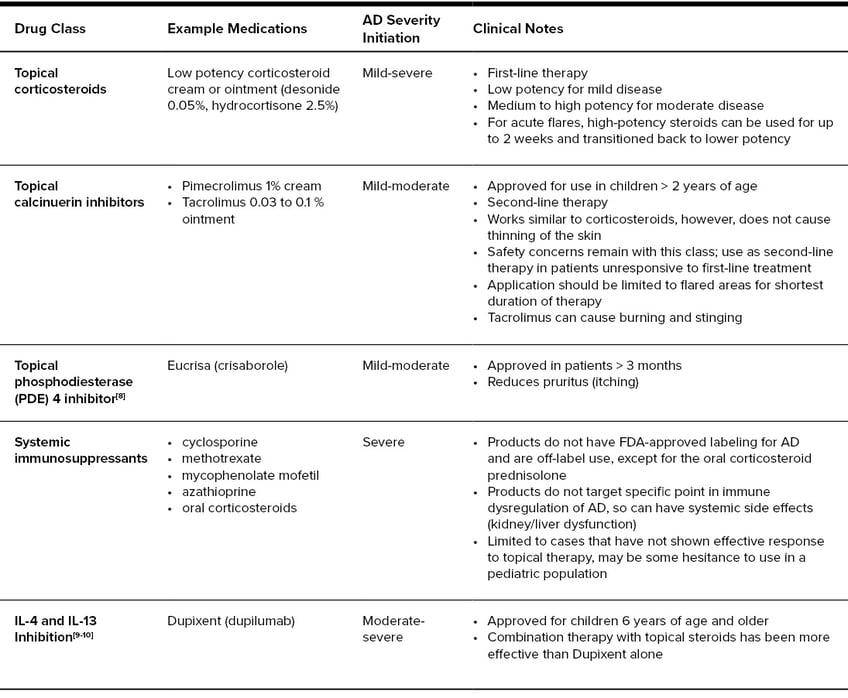
Unfortunately, sometimes avoidance and over-the-counter options are not enough and many AD sufferers have to start using prescription treatments to get their condition under control. First-line prescription treatments include topical corticosteroids, starting with low-medium potency, followed by topical calcinuerin inhibitors (TCI) or Eucrisa® (crisaborole). As the condition worsens or if it is considered severe, treatment can include phototherapy, Dupixent® (dupilumab), the first biologic approved for the treatment of AD, or systemic immunosupressants (cyclosporine, methotrexate, mycophenolate mofetil, azathioprine or oral corticosteroids).[2, 5] Systemic immunosupressants can have side effects or be difficult to tolerate.
Current Prescription Therapy Options for Atopic Dermatitis[6, 7]
Atopic Dermatitis Treatment Options in the Pipeline: Atopic dermatitis is associated with an immune system response. It primarily involves interleukin (IL) cytokines, which are secreted by immune cells and may be inflammatory. Specifically, janus kinase (JAK) activation may be involved in signaling more than 50 cytokines, including IL-4, IL-5, IL-13 and IL-31 . Many of the medications in the development pipeline for the treatment of AD utilize a mechanism of action that targets these cytokines and JAK enzymes.[11, 12]
There are already approved JAK inhibitors on the market for the treatment of other diseases, such as rheumatoid arthritis, graft-versus-host disease, myelofibrosis and ulcerative colitis. It is hypothesized that JAK inhibition not only modifies the body’s immune response, but possibly improves the skin as well. JAK inhibitors are not biologics; they are disease-modifying agents approved through the FDA new drug application (NDA) process. Recently, JAK inhibitors’ benefit-risk profile has been under scrutiny, including their long-term safety profile, which could determine much of the upcoming FDA reviews for these pipeline AD oral products.
Tralokinumab and lebrikizumab are drugs in the pipeline that selectively target IL-13 inhibition, similar to Dupixent. The first IL-31 inhibitor, nemolizumab, is also in the pipeline. IL-31 is known as the “itch cytokine.” Nemolizumab blocks IL-31 signaling on effector cells and in peripheral neurons.[6]
Atopic Dermatitis Pipeline Comparison[11, 13-18]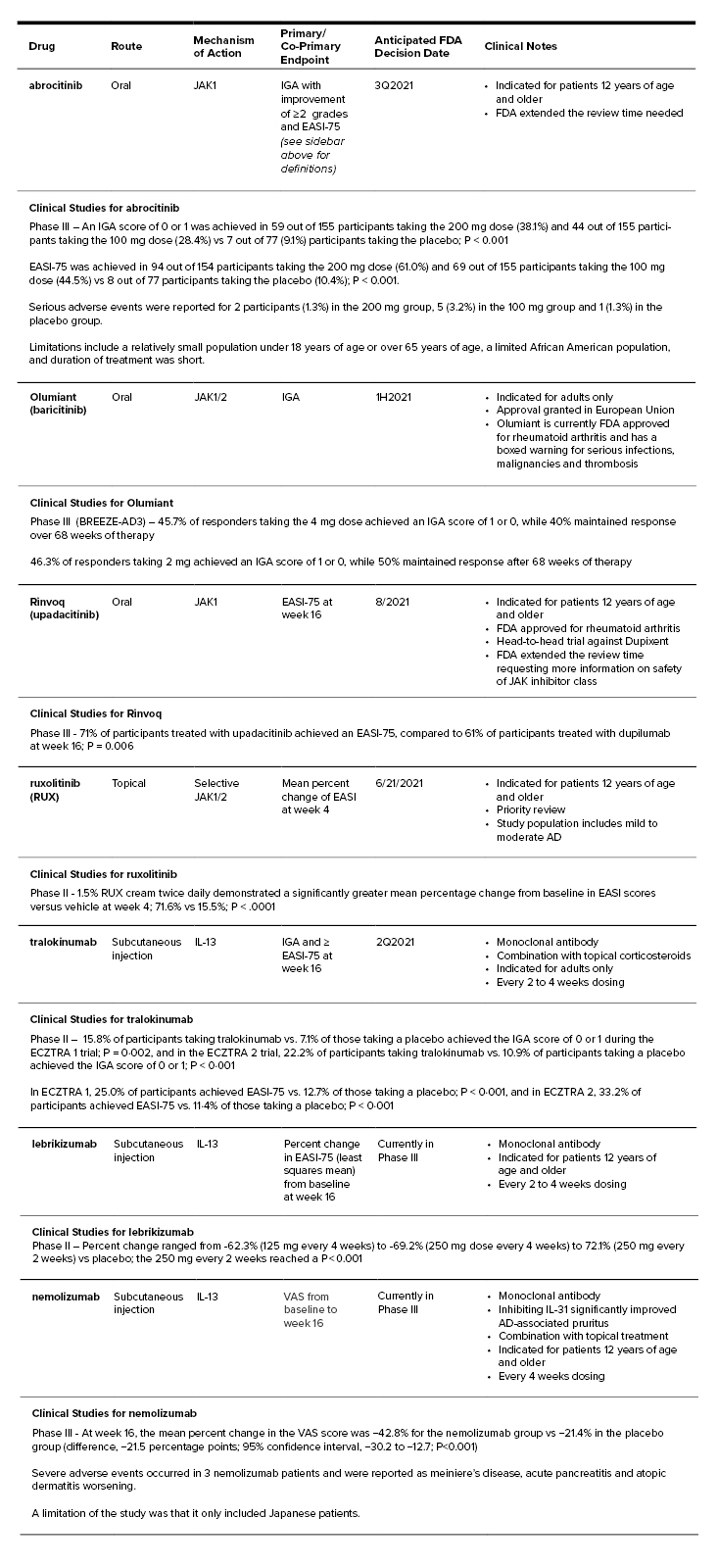
Primary Endpoint Definitions
- Investigator Global Assessment (IGA) Repose - Clear (0) or almost clear (1)
- Eczema Area and Severity Index score (EASI-75) - The proportion of participants achieving at least 75% improvement at week 12
- Visual-Analogue Scale (VAS) score for pruritus - Range of 0 to 100, with higher scores indicating worse pruritus
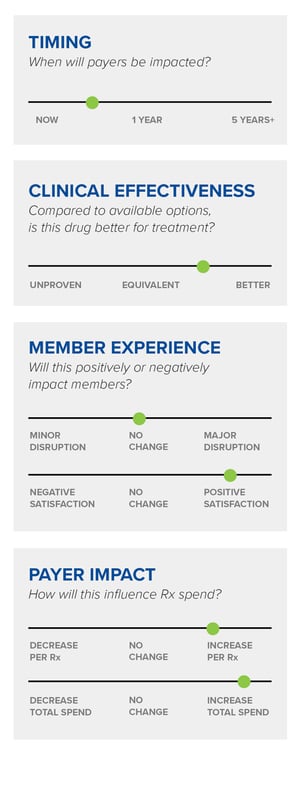
Impact to the Pharmacy Care Experience
Pipeline Monitoring: Elixir is closely monitoring this drug pipeline, which mainly focuses on moderate to severe AD.
Pharmacy & Therapeutics Review and Formulary Strategies: Elixir’s Pharmacy & Therapeutics (P&T) committee, which helps determine a drugs formulary placement, will rigorously review each future FDA approval to assure clinically appropriate, safe and efficacious products are provided on the formulary.
The safety profile of JAK inhibitors may be paramount in the FDA approval of any pipeline products for AD and will be something to review. Additionally, IL-13 inhibitors will want to prove comparable or superior to Dupixent’s already established safety and efficacy, as well as potentially offer an easier administration (such as oral or less frequent dosing) and a better value profile. It may take some time before true competition drives market share away from Dupixent when moderate to severe AD requires an immune suppressant. IL-31 inhibitor, nemolizumab, may find its place for patients experiencing severe itching not resolvable by current treatments. Elixir will conduct a value assessment of current-market products after clinical review.
Utilization Management: Utilization management, such as prior authorization, may be needed on pipeline medications to ensure clinically appropriate, first-line treatments were tried and that AD severity is met.
Payer Action Plan
Monitor the Drug Pipeline: At this time, there is no action that payers need to take. Elixir will continue to monitor the AD drug pipeline and keep our clients apprised of updates. Our P&T committee will review any newly approved FDA products and update clients when these products may be available for member utilization.
1] National Eczema Association. Eczema Stats. https://nationaleczema.org/research/eczema-facts/.
[2] Eichenfield, L. F., et al (2014). Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. Journal of the American Academy of Dermatology, 70(2), 338–351. https://doi.org/10.1016/j.jaad.2013.10.010
[3] Balma-Mena, A., et al. (2011). Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. International journal of dermatology, 50(6), 682–688. https://doi.org/10.1111/j.1365-4632.2010.04751.x
[4] Kim, J. P., Chao, L. X., Simpson, E. L., & Silverberg, J. I. (2016). Persistence of atopic dermatitis (AD): A systematic review and meta-analysis. Journal of the American Academy of Dermatology, 75(4), 681–687.e11. https://doi.org/10.1016/j.jaad.2016.05.028
[5] Boguniewicz, M., et al. (2018). Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology, 120(1), 10–22.e2. https://doi.org/10.1016/j.anai.2017.10.039
[6] Ständer S. (2021). Atopic Dermatitis. The New England journal of medicine, 384(12), 1136–1143. https://doi.org/10.1056/NEJMra2023911
[7] Sidbury, R., et al. (2014). Guidelines of care for the managment of atopic dermatitis. Journal of the American Academy of Dermatology. https://doi.org/10.1016/j.jaad.2014.03.030.
[8] Eucrisa Ointment 2% (crisaborole) [prescribing information]. New York, NY: Pfizer Labs; April 2020.
[9] Tameez Ud Din, A., Malik, I., Arshad, D., & Tameez Ud Din, A. (2020). Dupilumab for Atopic Dermatitis: The Silver Bullet We Have Been Searching for?. Cureus, 12(4), e7565. https://doi.org/10.7759/cureus.7565
[10] Dupixent (dupilumab) [prescribing information]. Tarrytown, NY: Regeneron Pharmaceuticals; June 2020.
[11] Kim, B. S., et al. (2020). Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. The Journal of allergy and clinical immunology, 145(2), 572–582. https://doi.org/10.1016/j.jaci.2019.08.042
[12] Renert-Yuval, Y., & Guttman-Yassky, E. (2020). New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology, 124(1), 28–35. https://doi.org/10.1016/j.anai.2019.10.005
[13] Silverberg, J. I., et al. (2020). Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA dermatology, 156(8), 863–873. https://doi.org/10.1001/jamadermatol.2020.1406
[14] EADV 2020: Lilly and Incyte Showcase New Data for Baricitinib for the Treatment of Moderate to Severe Atopic Dermatitis. Eli Lilly and Company (2020, October). Lilly Investors. Retrieved April 08, 2021, from https://investor.lilly.com/news-releases/news-release-details/eadv-2020-lilly-and-incyte-showcase-new-data-baricitinib
[15] RINVOQ™ (upadacitinib) Achieved Superiority Versus DUPIXENT® (dupilumab) For Primary and All Ranked Secondary Endpoints in Phase 3b Head-to-Head Study in Adults with Atopic Dermatitis. (2020 December). Abbvie News Center. Retrieved April 08, 2021 from https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-achieved-superiority-versus-dupixent-dupilumab-for-primary-and-all-ranked-secondary-endpoints-in-phase-3b-head-to-head-study-in-adults-with-atopic-dermatitis.htm
[16] Wollenberg, A., et al (2021). Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). The British journal of dermatology, 184(3), 437–449. https://doi.org/10.1111/bjd.19574
[17] Guttman-Yassky, E., et al. (2020). Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA dermatology, 156(4), 411–420. https://doi.org/10.1001/jamadermatol.2020.0079
[18] Kabashima, K., Matsumura, T., Komazaki, H., Kawashima, M., & Nemolizumab-JP01 Study Group (2020). Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. The New England journal of medicine, 383(2), 141–150. https://doi.org/10.1056/NEJMoa1917006


